Mon-Fri 9am-6pm
Compared to the standard of care
Reduced Mortality
Improve blood pressure
Reduce blood lactate level
Improved ARDS
The New Paradigm in Hypovolemic Shock Management.
Lyfaquin® injection is a sterile preparation of 1.0 mg centhaquine citrate intended for intravenous use. The product is available as 10 mL amber tubular glass vial. It is soluble in sodium chloride Injection I.P. (0.9% w/v). The active ingredient centhaquine citrate is white crystalline powder with empirical formula C28H33N3O7 and molecular weight of 523.58. The chemical name of centhaquine citrate is, 2-[2-[4-(3-Methylphenyl)-1-piperazinyl]ethyl] quinoline citrate. Clinical study confirms that Lyfaquin® has a wide safety margin and the therapeutic dose is standardized as 0.01 mg / Kg body weight.
ABOUT USKey Improvement over Standard of Care
Stimulates α2B adrenergic receptors
Acts on α2B adrenergic receptors to produce venous constriction, increase venous return, increase cardiac output.
Stimulates α2A adrenergic receptors
Acts on central α2A adrenergic receptors to reduce sympathetic drive, decrease systemic vascular resistance and improve tissue blood perfusion.
Reduced Mortality
Significantly reduced mortality in hypovolemic shock
Improved ARDS
Improved ARDS and organ functions (MODS); Key divers of mortality
Reduced average time on hospital stay
Reduced average time on ventilators, ICU, & hospital stay of seriously ill patients.
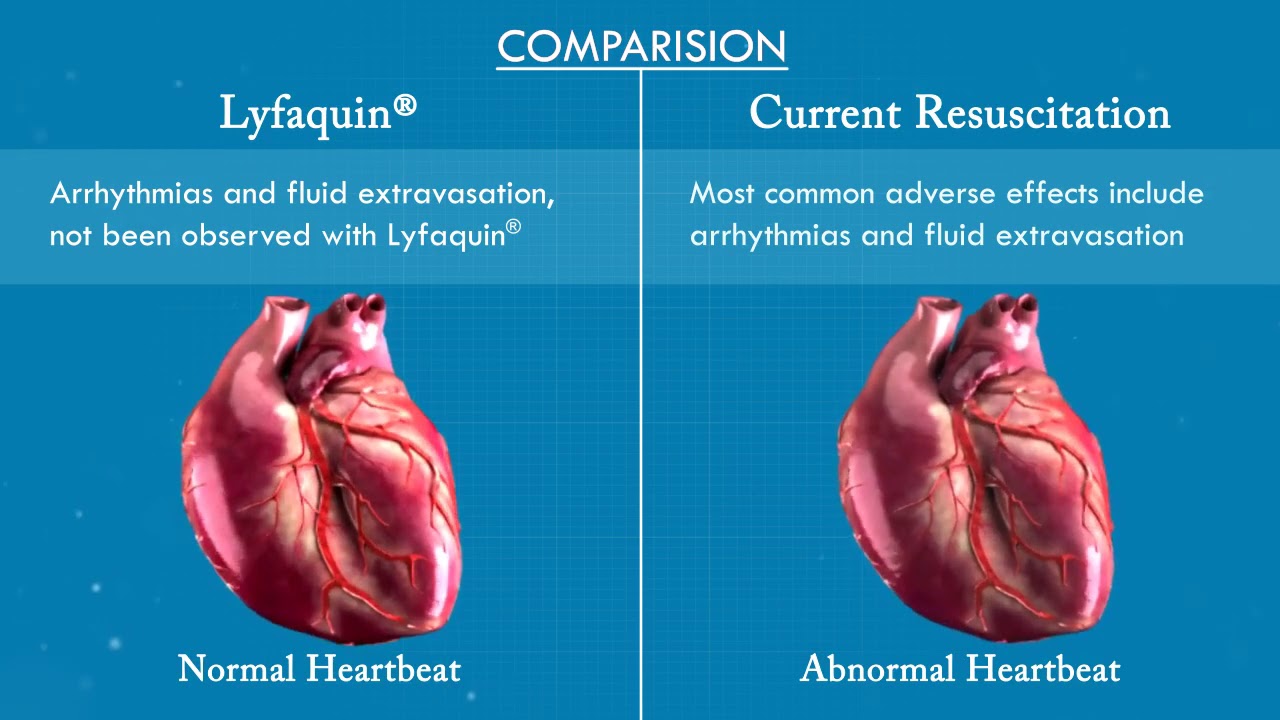
No serious adverse events
Well tolerated in clinical studies, no serious adverse events
Product Information
| Therapeutic indication | Hypovolemic Shock; as an adjuvant to standard of care |
| Dose | 0.01 mg/kg body weight (Reconstitution in normal saline) |
| Intravenous infusion rate | 100 mL in 1 hour |
| Dosing interval | Minimum 4 hours (Time gap between two doses not less than 4 hours) |
| Total doses in a day | 3 doses (Not more than 3 doses in a day/24 hours) |
| Maximum doses | 6 doses (Not more than 6 doses in 2 days/48 hours) |
Important safety information
Contraindications:
Hypersensitivity to Centhaquine or to any of the excipients
Special warnings and precautions for use:
Lyfaquin® administration with precautions in hepatic failure, renal failure and decompensated heart failure patient as safety and efficacy of Lyfaquin® not established in the same cases
The safety and efficacy of Lyfaquin® is also not established in pregnancy, lactating women, pediatric and geriatric population.
Drugs interactions:
No drug-drug interaction or drug-food interaction observed